
Chemistry, 03.12.2019 02:31 madivassallo
What is the purpose of adding phenolphthalein to your erlenmeyer flask prior to starting a titration?
a. the phenolphthalein acts as a color changing indicator to signal the endpoint of the reaction.
b. the phenolphthalein will change color once we have added the correct amount of base to our acid.
c. the phenolphthalein acts as an acid in the neutralization reaction.
d. the phenolphthalein acts as a base in the neutralization reaction.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:30
When svante arrhenius first proposed his acid-base theory, he was a doctoral candidate. his professors thought his ideas were unfounded. within a decade, the arrhenius theory of acid-base was widely accepted and praised within the scientific world. arrhenius defined acids as compounds having ionizable hydrogen and bases as compounds with ionizable a) barium. b) hydronium. c) hydroxide. d) oxygen.
Answers: 3

Chemistry, 22.06.2019 19:30
Describe the forces both attractive and repulsive that occur as two atoms move closer together.
Answers: 1

Chemistry, 23.06.2019 16:30
Two like-charged particles are placed close to each other. how would the force of repulsion be affected if the charge on one of the particles is doubled and that on the other is reduced to half the original value?
Answers: 1

Chemistry, 23.06.2019 17:30
What amount in moles does 242 l of carbon dioxide occupy at 1.32 atm and 20 degrees c?
Answers: 2
You know the right answer?
What is the purpose of adding phenolphthalein to your erlenmeyer flask prior to starting a titration...
Questions







English, 13.08.2020 05:01





Mathematics, 13.08.2020 05:01







Mathematics, 13.08.2020 05:01

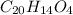 .
.


