Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:30
(1.6 × 10-19)(5.0 × 106) = c × 10d identify the missing numbers below to show the result of multiplying the numbers.
Answers: 1


Chemistry, 22.06.2019 12:40
Quiz1. which physical state of nitrogen has the highest entropy? a solid© b gasoc liquid
Answers: 1

Chemistry, 22.06.2019 22:30
Consider a culture medium on which only gram-positive organisms such as staphylococcus aureus colonies can grow due to an elevated nacl level. a yellow halo surrounds the growth, indicating the bacterium fermented a sugar in the medium, decreasing the ph as a result and changing the color of a ph indicator chemical. this type of medium would be referred to as a differential and enrichment culture.
Answers: 2
You know the right answer?
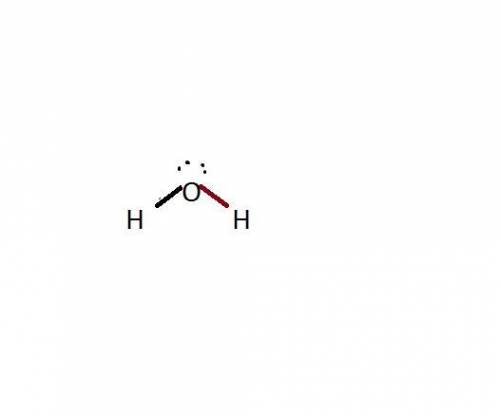
If a molecule has four hybrid sp3 orbitals, it can be concluded that the molecule has a
a: tr...
a: tr...
Questions


Mathematics, 31.10.2020 05:50

Biology, 31.10.2020 05:50

Chemistry, 31.10.2020 05:50


Chemistry, 31.10.2020 05:50

Mathematics, 31.10.2020 05:50


Mathematics, 31.10.2020 05:50



Mathematics, 31.10.2020 05:50



Mathematics, 31.10.2020 05:50

Advanced Placement (AP), 31.10.2020 05:50

Computers and Technology, 31.10.2020 05:50



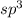 and the electronic geometry of the molecule will be tetrahedral.
and the electronic geometry of the molecule will be tetrahedral.
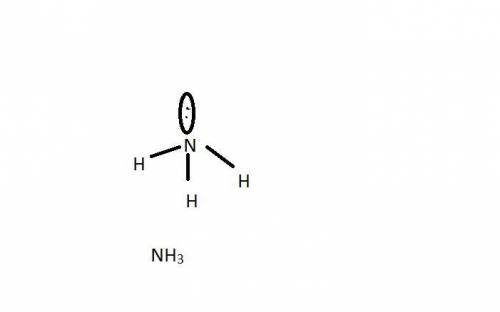
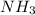 has nitrogen in
has nitrogen in 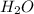 has oxygen in
has oxygen in