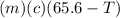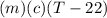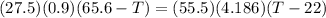Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:40
Kc = 0.040 for the system below at 450oc. if a reaction is initiated with 0.40 mole of cl2 and 0.40 mole of pcl3 in a 2.0 liter container, what is the equilibrium concentration of cl2 in the same system? pcl5(g) ⇄ pcl3(g) + cl2(g)
Answers: 3

Chemistry, 22.06.2019 07:00
Which atom or ion is the largest? a. k b. k+ c. ca d. ca2+ e. li
Answers: 1

Chemistry, 22.06.2019 10:10
Stage in which a typical star has completely stopped fusion
Answers: 1

Chemistry, 22.06.2019 14:50
Consider the following multistep reaction: a b→ab(slow) a ab→a2b(fast)−−−−−−−−−−−−−−−−− 2a b→a2b(overall) based on this mechanism, determine the rate law for the overall reaction. express your answer in standard masteringchemistry format. for example, if the rate law is k[a]3[b]2 type k*[a]^3*[b]^2
Answers: 3
You know the right answer?
A27.5 −g aluminum block is warmed to 65.6 ∘c and plunged into an insulated beaker containing 55.5 g...
Questions


Mathematics, 21.01.2022 06:10


Mathematics, 21.01.2022 06:10


English, 21.01.2022 06:10


Mathematics, 21.01.2022 06:10

Biology, 21.01.2022 06:10

Mathematics, 21.01.2022 06:10

Mathematics, 21.01.2022 06:10

Mathematics, 21.01.2022 06:10



Mathematics, 21.01.2022 06:10

Health, 21.01.2022 06:10


English, 21.01.2022 06:10