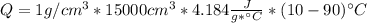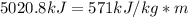Dry ice is solid carbon dioxide. instead of melting, solid carbon dioxide sublimes according to the following equation: co2(s)→co2(g). when dry ice is added to warm water, heat from the water causes the dry ice to sublime more quickly. the evaporating carbon dioxide produces a dense fog often used to create special effects. in a simple dry ice fog machine, dry ice is added to warm water in a styrofoam cooler. the dry ice produces fog until it evaporates away, or until the water gets too cold to sublime the dry ice quickly enough. suppose that a small styrofoam cooler holds 15.0 liters of water heated to 90 ∘c. calculate the mass of dry ice that should be added to the water so that the dry ice completely sublimes away when the water reaches 10 ∘c. assume no heat loss to the surroundings.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:30
This element exists in adundance in the sun.explain how you would go about capturing sunlight.would this captured sunlight contain any of the element?
Answers: 1

Chemistry, 23.06.2019 01:30
Will a solution form when the solvent and solute are both nonpolar? a. not likely b. never c. most likely
Answers: 1

Chemistry, 23.06.2019 04:00
If you are told to get 100 ml of stock solution to use to prepare smaller size sample for an experiment, which piece of glassware would you use?
Answers: 3

Chemistry, 23.06.2019 13:00
If volume remains the same while the mass of a substance the density of the substance
Answers: 1
You know the right answer?
Dry ice is solid carbon dioxide. instead of melting, solid carbon dioxide sublimes according to the...
Questions

Mathematics, 04.12.2020 07:00


Mathematics, 04.12.2020 07:00

English, 04.12.2020 07:00


History, 04.12.2020 07:00







Mathematics, 04.12.2020 07:00


Social Studies, 04.12.2020 07:00

Mathematics, 04.12.2020 07:00






 is the density
is the density