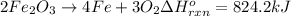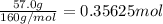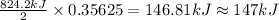Chemistry, 03.12.2019 03:31 dashavasilisk
The decomposition of 57.0 g of fe2o3 results in consider the following reaction. 2fe2o3 > 4fe + 3o2 deltah degree rxn = + 824.2 kj decomposition of 57.0 g of fe2o3 results in the release of 294 kj of heat. a. the absorption of 23500 kj of heat. b. the absorption of 147 kj of heat. c. the absorption of 294 kj of heat. d. the release of 23500 kj of heat. e. the release of 147 kj of heat.

Answers: 1


Another question on Chemistry



Chemistry, 22.06.2019 03:10
Between 2014 and 2016, more than 25,000 children in flint, michigan, drank water that was contaminated with lead from lead pipes. during this time, the city claimed the water was safe to drink. which of these actions could the city have taken to ensure that the drinking water was free from lead?
Answers: 3

Chemistry, 22.06.2019 06:00
24. a sports ball is inflated to an internal pressure of 1.85 atm at room temperature (25 °c). if the ball is then played with outside where the temperature is 7.5 °c, what will be the new pressure of the ball? assume the ball does not change in volume nor does any air leak from the ball a) 0.555 atm b) 1.74 atm c) 1.85 atm d) 1.97 atm
Answers: 2
You know the right answer?
The decomposition of 57.0 g of fe2o3 results in consider the following reaction. 2fe2o3 > 4fe +...
Questions

Mathematics, 06.04.2021 03:10

Mathematics, 06.04.2021 03:10



Mathematics, 06.04.2021 03:10

Mathematics, 06.04.2021 03:10

Mathematics, 06.04.2021 03:10

Physics, 06.04.2021 03:10


Mathematics, 06.04.2021 03:10



Mathematics, 06.04.2021 03:10

Social Studies, 06.04.2021 03:10



Physics, 06.04.2021 03:10