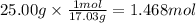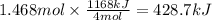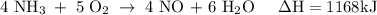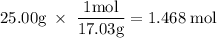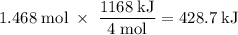Chemistry, 03.12.2019 04:31 averylivinglife2041
How much heat is absorbed/released when 25.00 g of nh3(g) reacts in the presence of excess o2(g) to produce no(g) and h2o(l) according to the following chemical equation? 4 nh3(g) + 5 o2(g) → 4 no(g) + 6 h2o(l) δh° = 1168 kj

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:30
Which of the following statements concerning the influence of culture on ethnic identity formation is accurate? a. one will reject ethnic identity if cultural stereotypes are encountered. b. if one’s ethnic city is different from the dominant cultural group, then one’s ethnic identity you will become weekend. c. if an the ethnic group is excepted by dominant culture, then ethnic identity formation can be a difficult process. d. similarity to the dominant culture can determine how easy it is for one to except ethnic differences.
Answers: 2

Chemistry, 22.06.2019 06:30
The following reaction shows sodium carbonate reacting with calcium hydroxide. na2co3 + ca(oh)2 → naoh + caco3 how many grams of naoh are produced from 20.0 grams of na2co3? (molar mass of na = 22.989 g/mol, c = 12.01 g/mol, o = 15.999 g/mol, ca = 40.078 g/mol, h = 1.008 g/mol) 12.2 grams 15.1 grams 24.4 grams 30.2 grams
Answers: 2

Chemistry, 23.06.2019 08:30
Kelly has come up with an explanation for why her sister is sometimes in a good mood and other times in a bad mood. she speculates that it is based on the hours of sleep her sister got the previous night. this explanation for her sister's behaviors is an example of a(n)
Answers: 3

Chemistry, 23.06.2019 19:30
There was a water with a boat and a rock in a boat. will the level of the water stay the same or higher or lower if u remove the rock from the boat and put it under water? ?
Answers: 3
You know the right answer?
How much heat is absorbed/released when 25.00 g of nh3(g) reacts in the presence of excess o2(g) to...
Questions

Biology, 29.08.2019 09:50


History, 29.08.2019 09:50




Mathematics, 29.08.2019 09:50


Chemistry, 29.08.2019 09:50

Biology, 29.08.2019 09:50


English, 29.08.2019 09:50

History, 29.08.2019 09:50





Social Studies, 29.08.2019 09:50

Business, 29.08.2019 09:50

Business, 29.08.2019 09:50