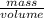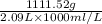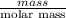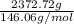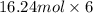Sulfur hexafluoride, a dense gas, is held in two separate containers in a storage room at an atmospheric pressure of 755 mmhg and 20.3 °c. the volume of container 1 is 2.09 l, and it contains 7.61 mol of the gas. the volume of container 2 is 4.46 l. determine the moles of f atoms in container 2 and the density of the gas at the conditions in the room

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 10:40
Asolid that forms and separates from a liquid mixture is called
Answers: 2

Chemistry, 22.06.2019 20:20
Nitric acid can be formed in two steps from the atmospheric gases nitrogen and oxygen, plus hydrogen prepared by reforming natural gas. in the first step, nitrogen and hydrogen react to form ammonia: (g) (g) (g) in the second step, ammonia and oxygen react to form nitric acid and water: (g) (g) (g) (g) calculate the net change in enthalpy for the formation of one mole of nitric acid from nitrogen, hydrogen and oxygen from these reactions. round your answer to the nearest .
Answers: 3

You know the right answer?
Sulfur hexafluoride, a dense gas, is held in two separate containers in a storage room at an atmosph...
Questions

Mathematics, 16.10.2019 23:00




English, 16.10.2019 23:00


History, 16.10.2019 23:00


Spanish, 16.10.2019 23:00

Chemistry, 16.10.2019 23:00

Computers and Technology, 16.10.2019 23:00


Mathematics, 16.10.2019 23:00

English, 16.10.2019 23:00

Physics, 16.10.2019 23:00





Mathematics, 16.10.2019 23:00

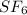 in container- 1 is as follows.
in container- 1 is as follows.