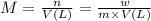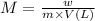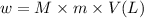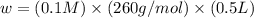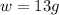Chemistry, 03.12.2019 07:31 jayjinks976
What mass of feso4^2- x 6h20 (molar mass=260g/mol) is required to produce 500 ml of a .10m iron (ii) sulfate solution.
a.) 9g
b.) 13g
c.) 36g
d.) 72g

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Three cards with holes are arranged in a straight line. a light is shined through the first card’s hole and travels through all three cards. what does this tell you about light rays? a) that light is reflected b) that light is refractive c) that light travels in a straight line d) that light does not travel in a straight line
Answers: 1

Chemistry, 22.06.2019 06:40
Three alkali metals in group 1 are a. calcium, strontium, barium b. boron, aluminum, gallium c. sodium, potassium, rubidium d. fluorine, iodine, chlorine
Answers: 1

Chemistry, 22.06.2019 08:30
Draw the skeletal structures of two different molecules that are each made of 5 carbon atoms and 12 hydrogen atoms.
Answers: 1

You know the right answer?
What mass of feso4^2- x 6h20 (molar mass=260g/mol) is required to produce 500 ml of a .10m iron (ii)...
Questions


Mathematics, 19.03.2020 00:30

Mathematics, 19.03.2020 00:30

English, 19.03.2020 00:30

Mathematics, 19.03.2020 00:30


Mathematics, 19.03.2020 00:30

Computers and Technology, 19.03.2020 00:30






Mathematics, 19.03.2020 00:30

Mathematics, 19.03.2020 00:30




English, 19.03.2020 00:30

Mathematics, 19.03.2020 00:31