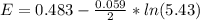Chemistry, 03.12.2019 17:31 adriana145
2co3+(aq)+2cl−(aq)→2co2+(aq)+cl2(g) . e∘=0.483 vwhat is the cell potential at 25 ∘c if the concentrations are [co3+]= 0.695 m , [co2+]= 0.175 m , and [cl−]= 0.315 m and the pressure of cl2 is pcl2= 8.50 atm ?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Asample of neon occupies a volume of 375 ml at stp. what will be the volume of neon if the pressure is reduced to 90.0 kpa? a. 422 ml b. 422 l c. 333 ml d. 333 l
Answers: 2

Chemistry, 21.06.2019 22:30
Often on a topographic map, every fifth contour line is darkened. what is this line called? a. key b.slope c.benchmark d. index contour
Answers: 1

Chemistry, 22.06.2019 12:40
In the following table, all the columns for the element calcium are filled out correctly. element electron structure of atom electron structure of ion net ionic charge calcium 1s22s22p63s23p64s2 1s32s22p63s23p64s1 +1 true false
Answers: 2

You know the right answer?
2co3+(aq)+2cl−(aq)→2co2+(aq)+cl2(g) . e∘=0.483 vwhat is the cell potential at 25 ∘c if the concentra...
Questions


Social Studies, 03.02.2021 21:20

Mathematics, 03.02.2021 21:20

Mathematics, 03.02.2021 21:20


Mathematics, 03.02.2021 21:20



Mathematics, 03.02.2021 21:20



Mathematics, 03.02.2021 21:20



History, 03.02.2021 21:20

Mathematics, 03.02.2021 21:20

Advanced Placement (AP), 03.02.2021 21:20



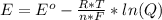
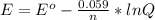
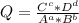
![Q=\frac{[Co^{+2}] ^{2}*P_{Cl2} }{[Co^{+3}] ^{2}*[Cl^{-}] ^{2} }](/tpl/images/0401/1816/09742.png)
![Q=\frac{[0.175M] ^{2}*8.5 }{[0.695M] ^{2}*[0.315M] ^{2} }](/tpl/images/0401/1816/998a7.png)