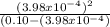Chemistry, 03.12.2019 17:31 alyssagibson470
A0.10 m solution of a weak monoprotic acid has a ph of 3.40 at 25°c. what is the acid-ionization
constant, ka, for this acid?
a) 1.6 x 10-6
b) 4.0 x 10-4
c) 3.4 x 10-5
d) 1.2 x 10-3
e) 1.8 x 10-7

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 06:30
How many moles of carbon dioxide will form if 2.5 moles of c3h8 is burned
Answers: 1

Chemistry, 23.06.2019 00:00
Before it was launched, a helium-filled balloon had a pressure of 201 kpa at a temperature of 27°c. at an altitude of 15,000 m, the pressure had decreased to 2.5 kpa and the temperature had dropped to -14 °c. the volume of the balloon increased to 59.3 m3. what is the original volume of the balloon? 13 m3 0.85 m3 0.077 m3 1.17 m3
Answers: 3

Chemistry, 23.06.2019 06:40
15. what volume of cci, (d = 1.6 g/cc) contain6.02 x 1025 cci, molecules (ci = 35.5)(1) 10.5 l(2) 250 ml(3) 9.625 l(4) 1.712 lplz answer with step by step explanation
Answers: 1
You know the right answer?
A0.10 m solution of a weak monoprotic acid has a ph of 3.40 at 25°c. what is the acid-ionization
Questions

English, 19.06.2020 05:57


History, 19.06.2020 05:57



Mathematics, 19.06.2020 05:57


Mathematics, 19.06.2020 05:57


Mathematics, 19.06.2020 05:57

Mathematics, 19.06.2020 05:57



History, 19.06.2020 05:57


Mathematics, 19.06.2020 05:57

Mathematics, 19.06.2020 05:57


Mathematics, 19.06.2020 05:57

Business, 19.06.2020 05:57

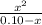
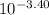 = 3.98 x 10⁻⁴
= 3.98 x 10⁻⁴