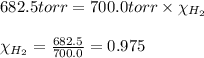Chemistry, 03.12.2019 18:31 taridunkley724
A30.0 ml sample of hydrogen gas (h2) is collected over water at 20.00∘c and has a total pressure of 700.0 torr. the partial pressure of water vapor at 20.00∘c is 17.5 torr. calculate the mole fraction of h2 gas in the sample.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:30
One mole of zinc has a mass of 65.4 grams. approximately how many atoms of zinc are present in one mole of zinc? 32 × 1023 atoms 6 × 1023 atoms 66 atoms 65 atoms
Answers: 1

Chemistry, 22.06.2019 02:30
Select each correct answer. more than one answer may be correct. which of the following is a characteristic of unicellular organisms? they can possess tissues and organs. all of their functions are performed by a single cell. they are usually microscopic. each of their cells is specialized to perform a specific function.
Answers: 1

Chemistry, 22.06.2019 13:00
6. using 3 – 4 sentences explain (in your own words) why water expands when it freezes? 7. using your knowledge of colligative properties explain whether sodium chloride or calcium chloride would be a more effective substance to melt the ice on a slick sidewalk. use 3 – 4 sentences in your explanation.
Answers: 1

Chemistry, 22.06.2019 13:30
Mary is conducting an experiment on how pollution affects plant growth. how can she ensure that her data are reliable?
Answers: 3
You know the right answer?
A30.0 ml sample of hydrogen gas (h2) is collected over water at 20.00∘c and has a total pressure of...
Questions

Social Studies, 20.05.2021 14:00

Mathematics, 20.05.2021 14:00



History, 20.05.2021 14:00

Computers and Technology, 20.05.2021 14:00

Health, 20.05.2021 14:00

History, 20.05.2021 14:00



Mathematics, 20.05.2021 14:00


Social Studies, 20.05.2021 14:00

Social Studies, 20.05.2021 14:00

English, 20.05.2021 14:00





Mathematics, 20.05.2021 14:00

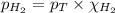
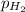 = partial pressure of hydrogen gas = 682.5 torr
= partial pressure of hydrogen gas = 682.5 torr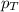 = total pressure = 700.0 torr
= total pressure = 700.0 torr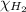 = mole fraction of hydrogen gas = ?
= mole fraction of hydrogen gas = ?