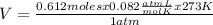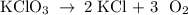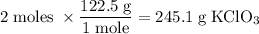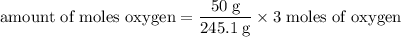Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Each of the following compounds contains a metal that can exhibit more than one ionic charge. provide systematic names for each of these compounds. (a) cr(clo3)6 (b) mo(cn)6 (c) cr2(so3)3 (d) v(clo2)2 (e) v(cn)5 (f) os(clo2)4
Answers: 3

Chemistry, 22.06.2019 12:30
What metric units would you use to measure the thickness of a key
Answers: 3

Chemistry, 22.06.2019 18:30
The table lists the lattice energies of some compounds.compoundlattice energy (kj/mol)lif –1,036licl –853naf –923kf –821nacl –786which statement about crystal lattice energy is best supported by the information in the table? the lattice energy increases as cations get smaller, as shown by lif and kf.the lattice energy increases as the cations get larger, as shown by lif and licl.the lattice energy decreases as cations get smaller, as shown by nacl and naf.the lattice energy decreases as the cations get smaller, as shown by naf and kf.
Answers: 3

Chemistry, 22.06.2019 22:50
At the current rate, a graph of carbon dioxide produced by fossil fuels over time would slope upward slope downward be horizontal be vertical
Answers: 3
You know the right answer?
Determine the volume of o2 (at stp) formed when 50.0 g of kclo3 decomposes according to the followin...
Questions

Mathematics, 12.06.2020 00:57



Mathematics, 12.06.2020 00:57

Mathematics, 12.06.2020 00:57




Mathematics, 12.06.2020 00:57

Mathematics, 12.06.2020 00:57


Mathematics, 12.06.2020 00:57

History, 12.06.2020 00:57







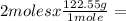 245.1 grams of KClO₃
245.1 grams of KClO₃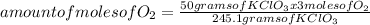
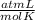 T= 0 C= 273 K
T= 0 C= 273 K