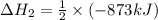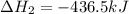The standard enthalpy change for the following reaction is 873 kj at 298 k.
2 kcl(s) 2 k(s) +...


Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:50
2. you__turn left on a red light if you are in the left-most lane of a one-way street, you're turning into the left-most lane of a one-way street, and no nearby sign prohibits the turn.
Answers: 2

Chemistry, 22.06.2019 12:00
What is the percentage of hydrogen in nitrogen trihydride
Answers: 1

Chemistry, 22.06.2019 12:10
If a molecule with a molecular formula of c13h18 is treated with an excess of h2 in the presence of finally divided pt metal under conditions required for maximum hydrogenation of the molecule to give a molecule with a formula c13h24, how many rings are in the molecule?
Answers: 3

Chemistry, 22.06.2019 20:00
I’m an electrically neutral atomic any element, there are equal numbers of
Answers: 2
You know the right answer?
Questions




SAT, 03.12.2021 23:30

English, 03.12.2021 23:30


Mathematics, 03.12.2021 23:30


History, 03.12.2021 23:30

Mathematics, 03.12.2021 23:30

Mathematics, 03.12.2021 23:30





History, 03.12.2021 23:30




Social Studies, 03.12.2021 23:30

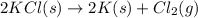
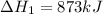
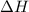 for the following reaction i.e,
for the following reaction i.e,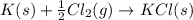
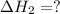
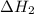 for the reaction will be:
for the reaction will be: