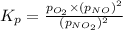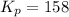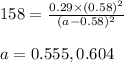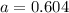Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:30
Aradio signal from a gps satellite take only about 0.067 seconds to reach a gps reciever. if the speed of light is about 300,000km/s, then approximately how far away is the reciever from from the satellite?
Answers: 1

Chemistry, 22.06.2019 11:40
Calculate the number of kilojoules to warm 125 g of iron from 23.5°c to 78.0°c.
Answers: 3

Chemistry, 22.06.2019 12:00
Under normal conditions, describe how increasing the temperatures effects the solubility of a typical salt
Answers: 1

Chemistry, 22.06.2019 13:30
Ants live on acacia trees in south america. the ants feed on sugars secreted by the trees. the trees provide room for the ants to live. the ants sting any other insect or animal that comes to eat the trees. what type of relationship is this?
Answers: 1
You know the right answer?
At 1000 k, a sample of pure no2 gas decomposes. 2 no2(g) equilibrium reaction arrow 2 no(g) + o2(g)...
Questions


Mathematics, 18.02.2021 20:00

Mathematics, 18.02.2021 20:00

Mathematics, 18.02.2021 20:00





Mathematics, 18.02.2021 20:00



Geography, 18.02.2021 20:00

Biology, 18.02.2021 20:00


Mathematics, 18.02.2021 20:00

Mathematics, 18.02.2021 20:00


Mathematics, 18.02.2021 20:00


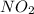 in the mixture is 0.58 atm and 0.024 atm respectively.
in the mixture is 0.58 atm and 0.024 atm respectively.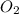 = 0.29 atm
= 0.29 atm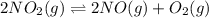
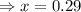
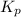 for above equation follows:
for above equation follows: