

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:20
Complete the table for ion charge based upon their losing or gaining electrons in the outer shell. (use the periodic table as necessary.) group most likely ionic charge # of valence electrons i +1 ii +2 iii +3 iv +4 or -4 v -3 vi -2 vii -1 viii 0
Answers: 2

Chemistry, 21.06.2019 18:30
For each of the following mixtures decide if filtering would be suitable to separate the substances. explain your answers. oil in water sugar in water sand in water chalk in water tea leaves in a cup of tea
Answers: 2

Chemistry, 22.06.2019 05:50
According to coulomb's law, how would the electrical force between particles change if the product of their electrical charge increased?
Answers: 1

Chemistry, 22.06.2019 16:40
The diagram below shows the movement of particles. what does this piece of evidence best support? the collision theory the maxwell-boltzmann distribution the effect of pressure on reaction rates the effect of temperature on reaction rates
Answers: 3
You know the right answer?
Calculate the mass of oxygen (in mg) dissolved in a 5.00 l bucket of water exposed to a pressure of...
Questions

Mathematics, 23.03.2021 04:20

English, 23.03.2021 04:20

Physics, 23.03.2021 04:20

English, 23.03.2021 04:20





Mathematics, 23.03.2021 04:20

Mathematics, 23.03.2021 04:20

Biology, 23.03.2021 04:20

Mathematics, 23.03.2021 04:20

English, 23.03.2021 04:20


History, 23.03.2021 04:20

Mathematics, 23.03.2021 04:20


Social Studies, 23.03.2021 04:20


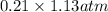
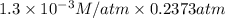
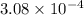 M
M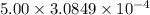
 mol
mol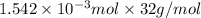 mol
mol 

