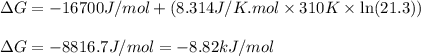Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:00
Which object forms when a supergiant runs out of fuel? a red giant a black hole a white dwarf a neutron star
Answers: 1

Chemistry, 22.06.2019 09:00
Which two scientific disciplines are bridged by pharmaceutical drugs? chemistry and forensics chemistry and medicine biology and forensics biology and criminology
Answers: 3


Chemistry, 22.06.2019 12:00
An atom's configuration based on its number of electrons ends at 3p4. another atom has seven more electrons. starting at 3p, what is the remaining configuration? 3p63d34s2 3p43d54s2 3p64s23d3 3p44s23d
Answers: 3
You know the right answer?
Consider the fructose-1,6-bisphosphatase reaction. calculate the free energy change if the ratio of...
Questions

Mathematics, 25.02.2021 16:20

Chemistry, 25.02.2021 16:20


Mathematics, 25.02.2021 16:20

Mathematics, 25.02.2021 16:20

History, 25.02.2021 16:20


Computers and Technology, 25.02.2021 16:20



Mathematics, 25.02.2021 16:20

English, 25.02.2021 16:20

History, 25.02.2021 16:20

Mathematics, 25.02.2021 16:20



Spanish, 25.02.2021 16:20


Mathematics, 25.02.2021 16:20

Mathematics, 25.02.2021 16:20

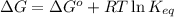
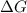 = free energy of the reaction
= free energy of the reaction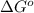 = standard Gibbs free energy = -16.7 kJ/mol = -16700 J/mol (Conversion factor: 1kJ = 1000J)
= standard Gibbs free energy = -16.7 kJ/mol = -16700 J/mol (Conversion factor: 1kJ = 1000J)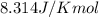
![37^oC=[273+37]K=310K](/tpl/images/0401/5815/c6b28.png)
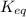 = Ratio of concentration of products and reactants = 21.3
= Ratio of concentration of products and reactants = 21.3