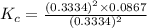Chemistry, 03.12.2019 21:31 maxi12312345
At a certain temperature, 0.760 mol so3 is placed in a 1.50 l container. 2so3(g) = 2so2(g) + o2(g)at equilibrium, 0.130 mol o2 is present. calculate kc.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:00
Given sno2 + 2h2 - sn + 2h20 tin oxide reacts with hydrogen to produce tin and water. how many moles of sno2 are needed to produce 500.0 grams of sn?
Answers: 3

Chemistry, 22.06.2019 13:30
If the concentration of phosphate in the cytosol is 2.0 mm and the concentration of phosphate in the surrounding fluid is 0.1 mm, how could the cell increase the concentration of phosphate in the cytosol? a) passive transportb) diffusionc) active transportd) osmosise) facilitated diffusion
Answers: 3


Chemistry, 22.06.2019 19:00
Sum of brother and sisters age is 26. four times the brothers age is subtracted from three times the sisters age, the difference is 8. what are the ages of the brother and sister?
Answers: 1
You know the right answer?
At a certain temperature, 0.760 mol so3 is placed in a 1.50 l container. 2so3(g) = 2so2(g) + o2(g)at...
Questions

Mathematics, 14.09.2021 01:00

Mathematics, 14.09.2021 01:00

English, 14.09.2021 01:00






Computers and Technology, 14.09.2021 01:00




Social Studies, 14.09.2021 01:00

Mathematics, 14.09.2021 01:00


English, 14.09.2021 01:00




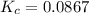
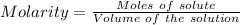
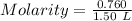
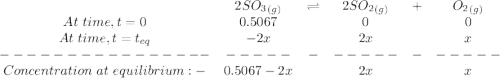
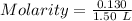
![K_c=\frac {[SO_2]^2[O_2]}{[SO_3]^2}](/tpl/images/0401/5810/3722c.png)