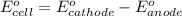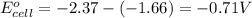Consider a galvanic cell based on the reaction al^3+_(aq) + mg_(s) rightarrow al_(s) + mg^2+ _(aq) the half-reactions are al^3+ + 3 e^- rightarrow al e degree = - 1.66 v mg^2+ + 2 e^- rightarrow mg e degree = - 2.37 v give the balanced cell reaction and calculate e degree for the cell.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:00
What happened in 2012 and how does it illustrate the importance of understanding the sun and how it works?
Answers: 3


Chemistry, 23.06.2019 00:30
There are approximately 15 milliliters (ml) in 1 tablespoon (tbsp). what expression can be used to find the approximate number of milliliters in 3 tbsp?
Answers: 1

Chemistry, 23.06.2019 01:00
Which description best characterization the motion of particles in a solid
Answers: 1
You know the right answer?
Consider a galvanic cell based on the reaction al^3+_(aq) + mg_(s) rightarrow al_(s) + mg^2+ _(aq) t...
Questions




Mathematics, 14.01.2021 16:00

Mathematics, 14.01.2021 16:00


Mathematics, 14.01.2021 16:00

Mathematics, 14.01.2021 16:00

Spanish, 14.01.2021 16:00

History, 14.01.2021 16:00

Mathematics, 14.01.2021 16:00

History, 14.01.2021 16:00

Mathematics, 14.01.2021 16:00


Mathematics, 14.01.2021 16:00


Social Studies, 14.01.2021 16:00



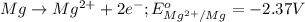 ( × 3)
( × 3)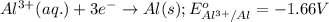 ( × 2)
( × 2)
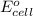 of the reaction, we use the equation:
of the reaction, we use the equation: