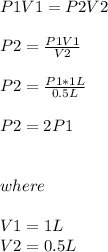Chemistry, 03.12.2019 23:31 lilybrok04
One liter of a gas is in a sealed chamber containing a moveable piston. if the piston is moved so that the volume of the gas is compressed to a volume of one-half liter, what will happen to the pressure on the gas? (assume the temperature is constant and no gas particles are lost.)
a)the pressure will be twice the original value.
b)the pressure will be half of the original value.
c)it would be impossible to move the piston since gases are not compressible.
d)the pressure will remain the same.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 06:00
In an investigation that uses the scientific method, which step immediately follows making a hypothesis? o summarizing the results o asking a question o making observations designing an experiment mark this and retum save and exit next submit
Answers: 2

Chemistry, 22.06.2019 07:30
Using data from seismic waves, geologists have learned that earth’s interior is made up of several
Answers: 1

Chemistry, 22.06.2019 09:00
How are isotopes of the same chemical element alike? how are they different?
Answers: 1
You know the right answer?
One liter of a gas is in a sealed chamber containing a moveable piston. if the piston is moved so th...
Questions


English, 03.12.2021 20:20

Geography, 03.12.2021 20:20








Business, 03.12.2021 20:20


Mathematics, 03.12.2021 20:20


Chemistry, 03.12.2021 20:20


Computers and Technology, 03.12.2021 20:20


Mathematics, 03.12.2021 20:20