
Chemistry, 03.12.2019 23:31 brittanyjacob8
The action of some commercial drain cleaners is based on the following reaction: 2 naoh(s) + 2 al(s) + 6 h2o(l) → 2 naal(oh)4(s) + 3 h2(g) what is the volume of h2 gas formed at stp when 6.32 g of al reacts with excess naoh?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:00
For ai it's atomic number is 13 and it's mass number is 27 how many neutrons does it have
Answers: 1

Chemistry, 22.06.2019 01:50
7. what temperature is need to just dissolve 50 g of nh4cl in 75 g of water? '
Answers: 1

Chemistry, 22.06.2019 04:30
Acamcorder has a power rating of 17 watts. if the output voltage from its battery is 7 volts, what current does it use?units:
Answers: 1

Chemistry, 22.06.2019 08:40
Write the formula for the following chemicals. 7. e. trinitrogen tetraoxide a calcium phosphate f. magnesium acetate b. potassium sulfide g nickel(iii) cyanide c carbon dioxide h. silver sulfate d. cobalt(ii) chloride
Answers: 1
You know the right answer?
The action of some commercial drain cleaners is based on the following reaction: 2 naoh(s) + 2 al(s...
Questions

Mathematics, 26.10.2020 23:00

Mathematics, 26.10.2020 23:00

Engineering, 26.10.2020 23:00

Mathematics, 26.10.2020 23:00




Physics, 26.10.2020 23:00



Social Studies, 26.10.2020 23:00

Mathematics, 26.10.2020 23:00

Biology, 26.10.2020 23:00


Mathematics, 26.10.2020 23:00




English, 26.10.2020 23:00

Mathematics, 26.10.2020 23:00

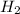 gas formed at STP is 7.86 liters.
gas formed at STP is 7.86 liters.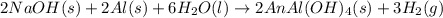
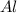 .
.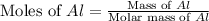
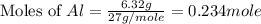
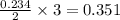 moles of
moles of 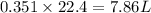 volume of
volume of 

