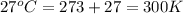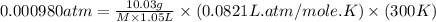Chemistry, 04.12.2019 00:31 zuleidysnegron
An aqueous solution of 10.03 g of catalase, an enzyme found in the liver, has a volume of 1.05 l at 27°c. the solution's osmotic pressure at 27°c is found to be 0.745 torr. calculate the molar mass of g/mol

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 16:40
Which of the following is a testable hypothesis? a. if i brush my teeth, i will get fewer cavities than if i don't brush my teeth. b. green toothpaste tastes better than blue toothpaste or red toothpaste. c. smart, careful, healthy people always brush their teeth. d. it's wrong to not brush your teeth before you have an important conversation with someone.
Answers: 1



Chemistry, 22.06.2019 12:30
The melting point of sulfur is 115 °c and its boiling point is 445 °c. what state would sulfur be in at 200 °c?
Answers: 1
You know the right answer?
An aqueous solution of 10.03 g of catalase, an enzyme found in the liver, has a volume of 1.05 l at...
Questions


Biology, 28.04.2021 01:20

Social Studies, 28.04.2021 01:20





Geography, 28.04.2021 01:20

Mathematics, 28.04.2021 01:20




Mathematics, 28.04.2021 01:20

Mathematics, 28.04.2021 01:20

History, 28.04.2021 01:20

Mathematics, 28.04.2021 01:20



History, 28.04.2021 01:20

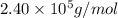
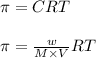
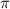 = osmotic pressure = 0.745 torr = 0.000980 atm (1 atm = 760 torr)
= osmotic pressure = 0.745 torr = 0.000980 atm (1 atm = 760 torr)