
Gaseous methane (ch4) reacts with gaseous oxygen gas (02) to produce gaseous carbon dioxide (co2) and gaseous water (h20). what is the theoretical yield of carbon dioxide formed from the reaction of 1.28 g of methane and 10.1 g of oxygen gas? be sure your answer has the correct number of significant digits in it. 02

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:30
In numbering carbon atoms in the parent chain of a hydrocarbon, why would you number from right to left, rather than left to right
Answers: 1

Chemistry, 22.06.2019 01:00
Water is important for the of cells. a: size, shape, and temperature b: temperature, color, and odor c: color, odor, and size d: shape, temperature, and color
Answers: 2

Chemistry, 22.06.2019 01:30
In a spacecraft, the following reaction occurs: co2(g) + 2lioh(s) -> lico3(s) + h2o(i) (i attached picture of equation) how many liters of carbon dioxide will 4 moles of lithium hydroxide (lioh) absorb? (one mole of any gads occupies 22.4 l under certain conditions of temperature and pressure. assume those conditions for this equation.) 45l 6.0l 3.0l 34l
Answers: 1

Chemistry, 22.06.2019 10:30
Which characteristics can be used to differentiate star systems? check all that apply.
Answers: 2
You know the right answer?
Gaseous methane (ch4) reacts with gaseous oxygen gas (02) to produce gaseous carbon dioxide (co2) an...
Questions

Mathematics, 07.04.2021 23:10

Mathematics, 07.04.2021 23:10

Mathematics, 07.04.2021 23:10


Mathematics, 07.04.2021 23:10



Mathematics, 07.04.2021 23:10

Mathematics, 07.04.2021 23:10


Mathematics, 07.04.2021 23:10



Mathematics, 07.04.2021 23:10

Arts, 07.04.2021 23:10


Mathematics, 07.04.2021 23:10


Mathematics, 07.04.2021 23:10

Spanish, 07.04.2021 23:10

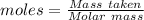
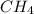 :-
:- 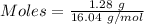
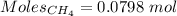
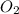 :-
:-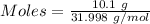
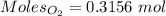
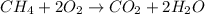
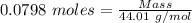
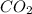 = 3.51 g
= 3.51 g

