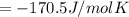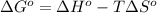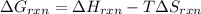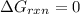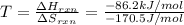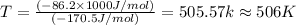Chemistry, 04.12.2019 01:31 feliciagraham14
At what temperature is the following reaction at equilibrium when all substances are at standard pressure?
assume that entropies and enthalpies of reaction do not vary with temperature.
pcl3(g) + cl2(g) pcl5(g) substance ∆hºf , kj mol–1 sº, j mol–1 k –1 pcl3(g) –288.7 311.6 cl2(g) 0 223.1 pcl5(g) –374.9 364.2 (a) 506 k (b) 1640 k (c) 1980 k (d) 4260 k

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 13:00
Jose and eric were given four samples in lab. the results of their analysis are shown in the table. based on the data they collected, which sample is most likely a metal?
Answers: 1

Chemistry, 22.06.2019 18:50
Asample of tin (ii) chloride has a mass of 0.49 g. after heating, it has a mass of 0.41 g. what is the percent by mass of water in the hydrate? %
Answers: 1

Chemistry, 22.06.2019 20:10
The lattice enthalpy (formation of ionic solid from ions in the gas phase) for agcl(s) is -916 kj/mol and the hydration enthalpy (dissolution of gaseous ions into water) is -850 kj/mol. how much heat (in joules) is involved in forming 1l of saturated agcl solution (1.8 × 10-4 g / 100 ml water) by dissolving agcl(s)? assume solution volume does not change much upon dissolution. the equations are given below. ag+(g) + cl−(g) æ agcl(s)
Answers: 3

You know the right answer?
At what temperature is the following reaction at equilibrium when all substances are at standard pre...
Questions

Mathematics, 30.10.2020 18:40


History, 30.10.2020 18:40

Mathematics, 30.10.2020 18:40

Mathematics, 30.10.2020 18:40

Chemistry, 30.10.2020 18:40



Mathematics, 30.10.2020 18:40

History, 30.10.2020 18:40

Social Studies, 30.10.2020 18:40




Health, 30.10.2020 18:40

Mathematics, 30.10.2020 18:40

Mathematics, 30.10.2020 18:40

Health, 30.10.2020 18:40

Mathematics, 30.10.2020 18:40

Physics, 30.10.2020 18:40

 gas =
gas = 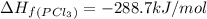
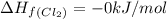
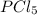 gas =
gas =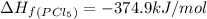
![\Delta H_{rxn}=\sum [n\times \Delta H_f(product)]-\sum [n\times \Delta H_f(reactant)]](/tpl/images/0402/0173/db29b.png)
![\Delta H_{rxn}=[(1\times \Delta H_f_{(PCl_5)})]-[(1\times \Delta H_f_{(PCl_3)})+(1\times \Delta H_f_{(Cl_2)})]](/tpl/images/0402/0173/51848.png)
![=[1\times (-374.9 kJ/mol)]-[1\times (-288.7 kJ/mol)+1\times 0 kJ/mol]](/tpl/images/0402/0173/d01dc.png)
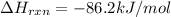
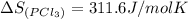
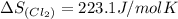
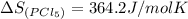
![\Delta S_{rxn}=\sum [n\times \Delta S_(product)]-\sum [n\times \Delta S_(reactant)]](/tpl/images/0402/0173/93994.png)
![\Delta S_{rxn}=[(1\times \Delta S_{(PCl_5)})]-[(1\times \Delta S_{(PCl_3)})+(1\times \Delta S_{(Cl_2)})]](/tpl/images/0402/0173/79ece.png)
![=[1\times 364.2 J/molK]-[1\times 311.6 J/mol K+1\times 223.1 J/mol K]](/tpl/images/0402/0173/813b0.png)