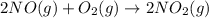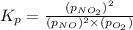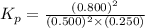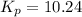Chemistry, 04.12.2019 01:31 Kingzion5775
For a gaseous reaction, standard conditions are 298 k and a partial pressure of 1 bar for all species. for the reaction
2 no ( g ) + o 2 ( g ) > 2 no 2 ( g )
the standard change in gibbs free energy is δ g ° = − 32.8 kj / mol . what is δ g for this reaction at 298 k when the partial pressures are:
pno = 0.500 bar , po2 = 0.250 bar , and pno 2 = 0.800 bar
deltag = ?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Which solution is an example of a nonelectrolyte? a. iodine in hexane b. sodium nitrate in waterc. acetic acid in waterd. hydrogen chloride in water
Answers: 2

Chemistry, 21.06.2019 22:30
Joseph has hypothesized that sound travels in waves. if he were following the scientific method, what should he do next? a. ask a question. b. test the hypothesis. c. study the results. d. tell other scientists about his hypothesis.
Answers: 1

Chemistry, 22.06.2019 12:30
Which element has the lowest electronegativity? calcium(ca) gallium(ga) selenium(se) bromine(br)
Answers: 1

Chemistry, 22.06.2019 17:30
What type of organic molecule comprises the majority of a potato?
Answers: 1
You know the right answer?
For a gaseous reaction, standard conditions are 298 k and a partial pressure of 1 bar for all specie...
Questions

Arts, 06.11.2020 23:50

Chemistry, 06.11.2020 23:50


Biology, 06.11.2020 23:50

Mathematics, 06.11.2020 23:50



Mathematics, 06.11.2020 23:50





Biology, 06.11.2020 23:50


Mathematics, 06.11.2020 23:50

Geography, 06.11.2020 23:50

Mathematics, 06.11.2020 23:50


Mathematics, 06.11.2020 23:50

History, 06.11.2020 23:50

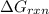 is, -27.0kJ/mole
is, -27.0kJ/mole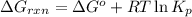 ............(1)
............(1)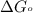 = standard Gibbs free energy = -32.8 kJ
= standard Gibbs free energy = -32.8 kJ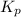 = equilibrium constant
= equilibrium constant