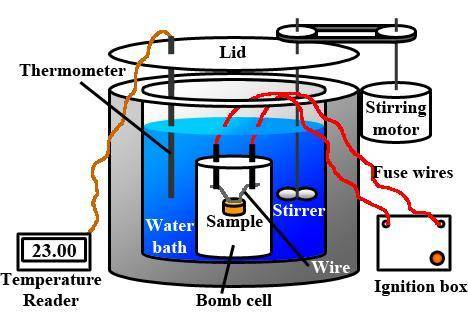
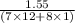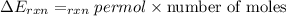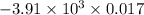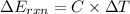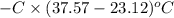When 1.550 g of liquid hexane (c6h14) undergoes combustion in a bomb calorimeter, the temperature rises from 25.87∘c to 38.13∘c. find δerxn for the reaction in kj/mol hexane. the heat capacity of the bomb calorimeter, determined in a separate experiment, is 5.73 kj/∘c. express your answer in kilojoules per mole to three significant figures.2. the combustion of toluene has a δerxn of –3.91×103 kj/mol. when 1.55 g of toluene (c7h8) undergoes combustion in a bomb calorimeter, the temperature rises from 23.12∘c to 37.57∘c. find the heat capacity of the bomb calorimeter. express the heat capacity in kilojoules per degree celsius to three significant

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:20
Which of these statements explains the difference between nuclear binding energy and the strong nuclear force ?
Answers: 3

Chemistry, 22.06.2019 22:00
All of the following are homogeneous mixtures except a) sugar dissolved in water. b) orange juice. c) coffee with cream. d) household vinegar. e) apple juice
Answers: 1

Chemistry, 23.06.2019 00:30
•hydration •dissociation •dissolving which one goes to which
Answers: 1

Chemistry, 23.06.2019 04:00
If you are told to get 100 ml of stock solution to use to prepare smaller size sample for an experiment, which piece of glassware would you use?
Answers: 1
You know the right answer?
When 1.550 g of liquid hexane (c6h14) undergoes combustion in a bomb calorimeter, the temperature ri...
Questions


Mathematics, 30.01.2021 05:10


Mathematics, 30.01.2021 05:10

Mathematics, 30.01.2021 05:10

English, 30.01.2021 05:10




Computers and Technology, 30.01.2021 05:10

Mathematics, 30.01.2021 05:10

Mathematics, 30.01.2021 05:10

English, 30.01.2021 05:10

Mathematics, 30.01.2021 05:10


Mathematics, 30.01.2021 05:10

Mathematics, 30.01.2021 05:10

Mathematics, 30.01.2021 05:10


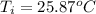 ,
, 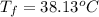
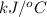
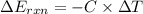
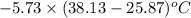
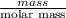
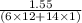
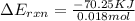
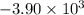 kJ/mol
kJ/mol
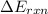 for the reaction in kJ/mol hexane is
for the reaction in kJ/mol hexane is