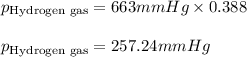Chemistry, 04.12.2019 01:31 andrejr0330jr
Amixture of nitrogen and hydrogen gases, at a total pressure of 663 mm hg, contains 3.46 grams of nitrogen and 0.156 grams of hydrogen. what is the partial pressure of each gas in the mixture? pn2 = mm hg ph2 = mm hg

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:40
Chemical kinetics what was the rate of reaction in trial 3? choose the closest answer.
Answers: 3

Chemistry, 22.06.2019 13:00
How many moles of sulfur dioxide are produced when 4.38 moles of oxygen completely react with iron (iv) sulfide
Answers: 2

Chemistry, 22.06.2019 17:00
What is the approximate vapor pressure when the gas condenses at 70 degrees celsius
Answers: 2

Chemistry, 22.06.2019 18:50
Which of the following is a conclusion that resulted from ernest rutherford’s scattering experiment? (will mark brainliest) a. the nucleus is negatively charged b. the atom is a dense solid and is indivisible c. the mass is conserved when atoms react chemically d. the nucleus is very small and the atom is mostly empty space
Answers: 3
You know the right answer?
Amixture of nitrogen and hydrogen gases, at a total pressure of 663 mm hg, contains 3.46 grams of ni...
Questions

Physics, 30.01.2021 07:20


Mathematics, 30.01.2021 07:20


Biology, 30.01.2021 07:20



Mathematics, 30.01.2021 07:20



Mathematics, 30.01.2021 07:20


Chemistry, 30.01.2021 07:20

Mathematics, 30.01.2021 07:20


Mathematics, 30.01.2021 07:20

Mathematics, 30.01.2021 07:20

Mathematics, 30.01.2021 07:20


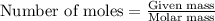
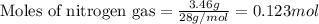
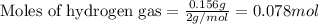
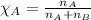 ......(1)
......(1)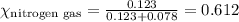
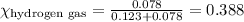
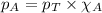 ......(2)
......(2)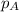 = partial pressure of substance A
= partial pressure of substance A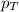 = total pressure = 663 mmHg
= total pressure = 663 mmHg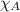 = mole fraction of substance A
= mole fraction of substance A