
Chemistry, 04.12.2019 02:31 dancer2814
Line spectra from all regions of the electromagnetic spectrum, including the paschen series of infrared lines for hydrogen, are used by astronomers to identify elements present in the atmospheres of stars. calculate the wavelength of the photon emitted when the hydrogen atom undergoes a transition from n = 5 to n = 3. (r = 2.179 x 10-18 j r = 1.096776 x 10^7 m-1) a. 205.1 nm b. 384.6 nm c. 683.8 nm d. 1282 nm e. > 1500 nm

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:00
Two friends at different locations want to communicate with each other by sending low energy signals. which of the following methods can they use to communicate? a) produce x-rays using colliding electrons and send them to radios, which capture sound b) send messages using infrared radiation, which travel in the form of waves c) send radio waves through intervening media like radio and television d) produce sound waves using microwaves from heated objects
Answers: 2

Chemistry, 21.06.2019 22:00
What is driving behind plate tectonics (plate movment)? a) gravity only b) inertia c) convection and gravity d) the sun theres no option for science so i picked chemistry. plz
Answers: 2

Chemistry, 22.06.2019 10:00
Suppose the universe were completely empty except for one object-a solid sphere moving through space of 100 km/s. what sort of path would the object be moving in? explain your answer
Answers: 1

You know the right answer?
Line spectra from all regions of the electromagnetic spectrum, including the paschen series of infra...
Questions




History, 10.12.2019 20:31


History, 10.12.2019 20:31





Mathematics, 10.12.2019 20:31

History, 10.12.2019 20:31





Spanish, 10.12.2019 20:31

Mathematics, 10.12.2019 20:31


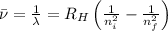
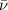 = Wave number
= Wave number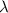 = Wavelength of radiation
= Wavelength of radiation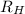 = Rydberg's Constant
= Rydberg's Constant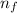 = Higher energy level
= Higher energy level 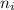 = Lower energy level
= Lower energy level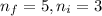
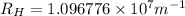
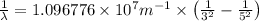
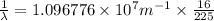
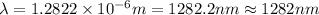
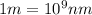 )
)


