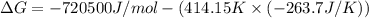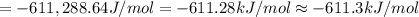Chemistry, 04.12.2019 03:31 ashcookie27
The value of delta g at 141.0 degrees celsius for the formation of phosphorous trichloride from its constituent elements,
p2(g) + 3cl2(g) > 2pcl3(g)
is kj/mol. at 25.0 degrees celsius for this reaction, delta h is -720.5 kj/mol, delta g is -642.9 kj/mol, and delta s is -263.7 j/k.
a.) -829.7
b.) 1.08 x 10^5
c.) 3.65 x 10^4
d.) -683.3
e.) -611.3

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Which is mostly likely why many scientists reject the cold fusion theory
Answers: 1

Chemistry, 22.06.2019 08:30
Which metal exist in liquid state and can be cut with knife ?
Answers: 2

Chemistry, 22.06.2019 10:50
Someone offer some answers to this, i will give 98 coins and mark as brainliest! i will put the rest of the lab down in the comments,solutions pre-lab questions: in this lab, you will make fruit drinks with powdered drink mix. complete the pre-lab questions to get the values you need for your drink solutions. calculate the molar mass of powered fruit drink mix, made from sucrose (c12h22o11).using stoichiometry, determine the mass of powdered drink mix needed to make a 1.0 m solution of 100 ml. (hint: use molarity = to find the moles of drink mix, then convert moles to grams using a mole conversion.)what mass of powdered drink mix is needed to make a 0.5 m solution of 100 ml?
Answers: 1

You know the right answer?
The value of delta g at 141.0 degrees celsius for the formation of phosphorous trichloride from its...
Questions

SAT, 14.01.2022 23:20




SAT, 14.01.2022 23:20

English, 14.01.2022 23:20

Mathematics, 14.01.2022 23:20

Computers and Technology, 14.01.2022 23:20





Mathematics, 14.01.2022 23:30


History, 14.01.2022 23:30



SAT, 14.01.2022 23:30


Mathematics, 14.01.2022 23:30