Chemistry, 04.12.2019 03:31 christophercordero15
Two nonpolar organic liquids, hexane (c6h14) and heptane (c7h16), are mixed. do you expect δhsoln to be a large positive number, a large negative number, or close to zero? fill in the following sentence: δhsoln is determined by the relative magnitudes of the "old" solute-solute (δhsolute) and solvent-solvent (δhsolvent) interactions and the "new" solute-solvent (δhmix) interactions. in this process, the energy of mixing hexane and heptane (δhmix) is the energy of separating them individually (δhsolute + δhsolvent), so δhsoln is expected to be .

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Suppose a lab group reports a ppercent yield of sand of 105. is it really possible to collect more sand than was originally represented? what is the possible explanation for the extra product?
Answers: 2

Chemistry, 22.06.2019 07:20
After watching the video "zinc strip in copper nitrate solution", and reading the instructions, click on the link labeled "start" just below the drawing of the pencil tip. follow the direction to complete the 3x3 grid. answer the below questions for the portion of the activity in which sn(s) is placed in agno3(aq)
Answers: 1

Chemistry, 22.06.2019 18:00
Heat is the total potential energy of a substance that can be transferred. true false
Answers: 1

You know the right answer?
Two nonpolar organic liquids, hexane (c6h14) and heptane (c7h16), are mixed. do you expect δhsoln to...
Questions



Mathematics, 04.11.2020 20:10

Mathematics, 04.11.2020 20:10

Mathematics, 04.11.2020 20:10





Mathematics, 04.11.2020 20:10

History, 04.11.2020 20:10



History, 04.11.2020 20:10

English, 04.11.2020 20:10

Mathematics, 04.11.2020 20:10

Mathematics, 04.11.2020 20:10


Computers and Technology, 04.11.2020 20:10

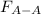 =
= 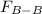 =
=