
The synthesis of methanol from carbon monoxide and hydrogen gas is described by the following chemical equation:
co(g)+2h2(g)⇌ch3oh(g)
the equilibrium constant for this reaction at 25 ∘c is kc=2.3×104. in this tutorial, you will use the equilibrium-constant expression to find the concentration of methanol at equilibrium, given the concentration of the reactants.
determine the expression for the equilibrium constant, kc, for the reaction by identifying which terms will be in the numerator and denominator:
the equilibrium-constant expression is a mathematical equation that can be rearranged to solve for any of the variables in it. rearrange the equilibrium-constant expression to solve for [ch3oh].
[ch3oh]=[ch3oh]=
1kc[co][h2]2
kc[co][h2]2
kc[co][h2]2
[co][h2]2kc

Answers: 3


Another question on Chemistry



Chemistry, 22.06.2019 19:20
Anyone who's in connections academy chemistry b have the factors that affect the rate of a reaction portfolio already done?
Answers: 3

Chemistry, 22.06.2019 22:30
The vapor pressure of ethanol is 1.00 × 102 mmhg at 34.90°c. what is its vapor pressure at 61.61°c? (δhvap for ethanol is 39.3 kj/mol.)
Answers: 2
You know the right answer?
The synthesis of methanol from carbon monoxide and hydrogen gas is described by the following chemic...
Questions


Mathematics, 18.02.2021 06:00


Mathematics, 18.02.2021 06:00


Spanish, 18.02.2021 06:00



Mathematics, 18.02.2021 06:00

English, 18.02.2021 06:00




Chemistry, 18.02.2021 06:00

Social Studies, 18.02.2021 06:00

Mathematics, 18.02.2021 06:00


Mathematics, 18.02.2021 06:00

Mathematics, 18.02.2021 06:00

![[CH_3OH]=K_c\times [CO]\times [H_2]^2](/tpl/images/0402/1764/fba08.png)
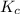
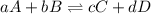
![K_{c}=\frac{[C]^c[D]^d}{[A]^a[B]^b}](/tpl/images/0402/1764/b6f47.png)
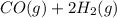 ⇌
⇌ 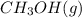
![K_{c}=\frac{[CH_3OH]}{[CO][H_2]^2}](/tpl/images/0402/1764/da8dd.png)


