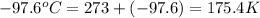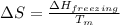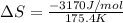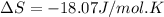Chemistry, 04.12.2019 04:31 cutebabyolivia
What is the entropy change for the freezing process of 1 mole of liquid methanol at its freezing temperature (–97.6˚c) and 1 atm? report your answer two points past the decimal with the unit j/molk. ∆h˚fus = 3.17 kj/mol.

Answers: 3


Another question on Chemistry



Chemistry, 22.06.2019 21:30
The solid xy decomposes into gaseous x and y: xy(s) m x(g) + y(g) kp = 4.1 (at 0 °c) if the reaction is carried out in a 22.4 l container, which initial amounts of x and y will result in the formation of solid xy?
Answers: 1

Chemistry, 23.06.2019 10:20
An engineer wishes to design a container that will hold 12.0 mol of ethane at a pressure no greater than 5.00x10*2 kpa and a temperature of 52.0 degrees celsius. what is the minimum volume the container can have?
Answers: 1
You know the right answer?
What is the entropy change for the freezing process of 1 mole of liquid methanol at its freezing tem...
Questions

History, 09.07.2019 14:10



Mathematics, 09.07.2019 14:10


History, 09.07.2019 14:10

Mathematics, 09.07.2019 14:10


Chemistry, 09.07.2019 14:10

History, 09.07.2019 14:10



History, 09.07.2019 14:10


English, 09.07.2019 14:10

Mathematics, 09.07.2019 14:10



History, 09.07.2019 14:10

Social Studies, 09.07.2019 14:10

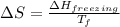
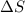 = change in entropy
= change in entropy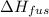 = change in enthalpy of fusion = 3.17 kJ/mol
= change in enthalpy of fusion = 3.17 kJ/mol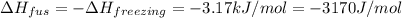
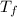 = freezing point temperature =
= freezing point temperature =