
Chemistry, 04.12.2019 04:31 tra10money
Classify each statement as true or false. drag the appropriate items to their respective bins. view available hint(s) reset the valence electrons of group 4a elements are in the 5s subshell. period 3 elements have an inner electron configuration of [ar). the highest principal quantum number of period 4 elements is 4 period 5 elements have six 4p electrons period 5 elements have an inner electron configuration of (kr group 8a elements have full outer principal s and p subshells. the valence electrons of group 3a elements are in an s and p subshell. the highest principal quantum number of period 4 elements is 5 false true submit

Answers: 2


Another question on Chemistry



Chemistry, 22.06.2019 16:00
Click the button that shows the correct relationship of the electron affinities of the elements sodium and phosphorus. sodium’s electron affinity value is more negative than the electron affinity value of phosphorus. phosphorus’ electron affinity value is more negative than the electron affinity value of sodium. this information cannot be determined using the periodic table. answer is b on e2020.
Answers: 3

Chemistry, 22.06.2019 16:20
When water dissolves sugar, which process is not involved? o dissociation o hydration o surface area of the solute increases sa
Answers: 1
You know the right answer?
Classify each statement as true or false. drag the appropriate items to their respective bins. view...
Questions

Arts, 12.05.2021 15:10

Mathematics, 12.05.2021 15:10



Chemistry, 12.05.2021 15:10





Mathematics, 12.05.2021 15:10

Mathematics, 12.05.2021 15:10

Physics, 12.05.2021 15:10



Mathematics, 12.05.2021 15:10



English, 12.05.2021 15:10

Geography, 12.05.2021 15:10

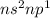 .
.

