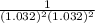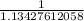Below regarding an electrochemical cell in an automotive lead-acid battery. the cell's anode is made of lead and the cathode is made of lead(iv) oxide. both are submerged in 4.30 m sulfuric acid. the half-reactions are: pbo_2(s) + 3h^+ (aq) + hso_4^- (aq) + 2e^- rightarrow pbso_4(s) + 2h_2o(l) e degree = 1.685 v pbso_4(s) + h^+(aq) + 2e^- rightarrow pb(s) + hso_4^- (aq) e degree = -0.356 v (a) calculate the value of e degree. (b) determine the initial value of e_cell. assume that the first ionization of h_2so_4 is complete and that [h^+] almostequalto [hso_4^-]. (c) find e_cell when the h^+ concentration has dropped by 76.00%. again, assume [h^+] almostequalto [hso_4^-].

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:00
The nuclear fission process releases neutrons and question 27 options: alpha particles electrons energy beta particles
Answers: 1

Chemistry, 22.06.2019 17:40
If 3 moles of a compound use 24 j of energy in a reaction, what is the a hreaction in j/mol?
Answers: 1

Chemistry, 22.06.2019 19:30
To calculate percent by mass, use the equation below: calculate the percent by mass of each element. %n = % %h = % %o = %
Answers: 3

Chemistry, 22.06.2019 22:10
Determine the ph of 0.10 m nh3 solution. nh3 is a weak base with a kb equal to 1.8 x 10-5 round answer to nearest whole number.
Answers: 1
You know the right answer?
Below regarding an electrochemical cell in an automotive lead-acid battery. the cell's anode is made...
Questions



English, 26.10.2020 14:20

English, 26.10.2020 14:20

Mathematics, 26.10.2020 14:20




Business, 26.10.2020 14:20



Geography, 26.10.2020 14:20



Mathematics, 26.10.2020 14:30



Physics, 26.10.2020 14:30

Mathematics, 26.10.2020 14:30

Medicine, 26.10.2020 14:30

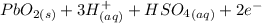 →
→ 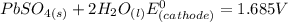
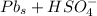 →
→ 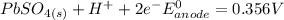
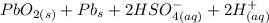 →
→ 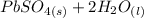
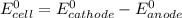
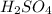 →
→ 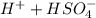
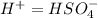 such that (;)
such that (;) 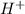 & b=
& b= 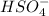
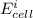 =
= 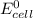 -
- 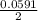 × log
× log 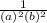
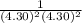
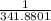
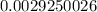
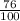
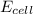 = 2.041V -
= 2.041V -