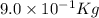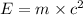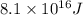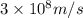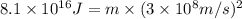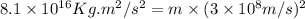Answers: 2


Another question on Chemistry

Chemistry, 20.06.2019 18:04
What is the smallest component or the most basic building block of any element ? a. an atom, b.a compound c.gas d.element
Answers: 1

Chemistry, 21.06.2019 17:30
You are performing an experiment in a lab to attempt a new method of producing pure elements from compounds. the only problem is that you do not know what element will form. by your previous calculations you know that you will have 6.3 moles of product. when it is complete, you weigh it and determine you have 604.4 grams. what element have you produced?
Answers: 1

Chemistry, 22.06.2019 06:30
Select the correct text in the passage. which sentences describe examples of sustainable living? i live in an old apartment building downtown, but my company is based in an office park on the outskirts of the city. i drive an old car that needs to be replaced. i plan to buy a hybrid for better gas mileage, but for now i am able to carpool with a couple of friends from work. the drive to the office park is about 45 minutes each way, but we do get to work in a modern building. the architects just received a leed certification for the design.
Answers: 3

Chemistry, 23.06.2019 03:30
Name atleast 3 type of energy associated with the microwave
Answers: 1
You know the right answer?
In a nuclear reaction, the energy released is equal to 8.1 x 1016 joules. calculate the mass lost in...
Questions

Mathematics, 10.04.2020 00:57

Mathematics, 10.04.2020 00:57


Mathematics, 10.04.2020 00:57

Mathematics, 10.04.2020 00:57



Mathematics, 10.04.2020 00:57

Mathematics, 10.04.2020 00:57

Mathematics, 10.04.2020 00:57


Mathematics, 10.04.2020 00:57

Mathematics, 10.04.2020 00:57



Physics, 10.04.2020 00:57