
Chemistry, 04.12.2019 06:31 cbrpilot1151
Isopropyl alcohol is mixed with water to produce a 40.0 % (v/v) alcohol solution. how many milliliters of each component are present in 675 ml of this solution? assume that volumes are additive.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:00
Calculate the mass of silver needed to react with chlorine to produce 126g if silver chloride?
Answers: 3

Chemistry, 22.06.2019 12:30
The bond energy for the van der waals bond between two helium atoms is 7.9×10−4ev. assuming that the average kinetic energy of a helium atom is (3/2)kbt, at what temperature is the average kinetic energy equal to the bond energy between two helium atoms
Answers: 1


Chemistry, 22.06.2019 21:30
Isopropyl alcohol, (ch3)2choh, is a common solvent. determine the percent by mass of hydrogen in isopropyl alcohol. a) 6.71% h b) 13.4% h c) 25.0% h d) 53.3% h
Answers: 1
You know the right answer?
Isopropyl alcohol is mixed with water to produce a 40.0 % (v/v) alcohol solution. how many millilite...
Questions


Physics, 05.05.2020 07:00

Mathematics, 05.05.2020 07:00


Mathematics, 05.05.2020 07:00

History, 05.05.2020 07:00


Mathematics, 05.05.2020 07:00




Social Studies, 05.05.2020 07:00








Mathematics, 05.05.2020 07:00

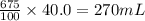 of alcohol
of alcohol

