
Chemistry, 04.12.2019 06:31 jaedenevan062907
Which of the following statements explain why the van der waals equation must be used to describe real gases? x. interactions between gas molecules reduces the temperature of the gas in the sample y. the non-zero volumes of gas particles effectively decrease the amount of "empty space" between them z. the molecular attractions between particles of gas decreases the pressure exerted by the gas

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:50
Ase your answer to this question on the information below.hydrocarbons and fissionable nuclei are among the sources used for the production of energy in the united states. a chemical reaction produces much less energy than a nuclear reaction per mole of reactant.the balanced chemical equation below represents the reaction of one molecule of a hydrocarbon with two molecules of oxygen.chemical equation: ch4 + 2o2 → co2 + 2h2o + 1.48 × 10−18 jthe nuclear equation below represents one of the many possible reactions for one fissionable nucleus. in this equation, x represents a missing product.nuclear equation: write an isotopic notation for the missing product represented by x in the nuclear equation.
Answers: 1

Chemistry, 22.06.2019 12:30
Avariable that is not being directly tested during an experiment should be changed varied experimented controlled
Answers: 1

Chemistry, 23.06.2019 04:30
Two liquids are poured into a beaker. after a few seconds, the beaker becomes warm. which of the following best describes this reaction? a. an exothermic reaction b. a decomposition reaction c. an endothermic reaction d. a single-displacement reaction
Answers: 1

Chemistry, 23.06.2019 04:31
Areaction is first order. if the initial reactant concentration is 0.0200 m, and 25.0 days later the concentration is 6.25 x 10-4 m, then its half-life is:
Answers: 1
You know the right answer?
Which of the following statements explain why the van der waals equation must be used to describe re...
Questions




Spanish, 23.10.2020 23:00

History, 23.10.2020 23:00

Mathematics, 23.10.2020 23:00

Arts, 23.10.2020 23:00


Chemistry, 23.10.2020 23:00

Mathematics, 23.10.2020 23:00

Spanish, 23.10.2020 23:00

Mathematics, 23.10.2020 23:00




Business, 23.10.2020 23:00

Physics, 23.10.2020 23:00



History, 23.10.2020 23:00

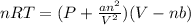 (1)
(1)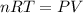 (2)
(2)


