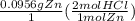Chemistry, 04.12.2019 07:31 tobywaffle1234
Zinc reacts with hydrochloric acid according to the reaction equation zn ( s ) + 2 hcl ( aq ) ⟶ zncl 2 ( aq ) + h 2 ( g ) how many milliliters of 5.50 m hcl (aq) are required to react with 6.25 g zn (s) ?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:10
Imagine that you have produced several versions of lactase, each of which differs from normal lactase by a single amino acid. describe a test that could indirectly determine which of the versions significantly alters the three-dimensional shape of the lactase protein.
Answers: 2

Chemistry, 22.06.2019 16:00
Which factor is likely to impact the possible number of compounds ?
Answers: 1

Chemistry, 22.06.2019 17:10
)benzene and toluene form nearly ideal solutions. consider an equimolar solution of benzene and toluene. at 20 °c the vapour pressures of pure benzene and toluene are 9.9 kpa and 2.9 kpa, respectively. the solution is boiled by reducing the external pressure below the vapour pressure. calculate (i) the pressure when boiling begins, (ii) the composition of each component in the vapour, and (iii) the vapour pressure when only a few drops of liquid remain. assume that the rate of vaporization is low enough for the temperature to remain constant at 20 °c.
Answers: 1

Chemistry, 22.06.2019 19:30
Estimate the molar mass of the gas that effuses at 1.6 times the effusion rate of carbon dioxide.
Answers: 1
You know the right answer?
Zinc reacts with hydrochloric acid according to the reaction equation zn ( s ) + 2 hcl ( aq ) ⟶ zncl...
Questions

Physics, 06.10.2020 18:01

Arts, 06.10.2020 18:01

English, 06.10.2020 18:01

Mathematics, 06.10.2020 18:01


Mathematics, 06.10.2020 18:01

History, 06.10.2020 18:01





Mathematics, 06.10.2020 18:01


Biology, 06.10.2020 18:01



History, 06.10.2020 18:01


Mathematics, 06.10.2020 18:01