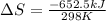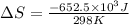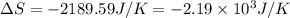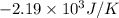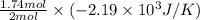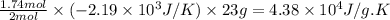Chemistry, 04.12.2019 07:31 pheonixhowls
Consider the reaction 2na(s) + 2h2o(l)2naoh(aq) + h2(g) using standard thermodynamic data at 298k, calculate the entropy change for the surroundings when 1.74 moles of na(s) react at standard conditions. s°surroundings = j/k g

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Which of the following best explains why the end of a spoon sticking out of a cup of hot water also gets hot? question 7 options: the heat from the hot water is conducted through the spoon handle the hot water heats the air surrounding the upper part of the spoon. the hot water causes a physical change in the spoon handle. the hot water causes a chemical reaction to take place in the spoon.
Answers: 2

Chemistry, 22.06.2019 06:00
When a spring is compressed, the energy changes from kinetic to potential. which best describes what is causing this change?
Answers: 3


Chemistry, 22.06.2019 10:00
Water's surface tension and heat storage capacity are accounted for by its a) orbitals b) weight c) hydrogen bonds d) mass e) size
Answers: 2
You know the right answer?
Consider the reaction 2na(s) + 2h2o(l)2naoh(aq) + h2(g) using standard thermodynamic data at 298k, c...
Questions


Mathematics, 22.06.2019 10:30


Chemistry, 22.06.2019 10:30


Computers and Technology, 22.06.2019 10:30

Social Studies, 22.06.2019 10:30

Chemistry, 22.06.2019 10:30



Mathematics, 22.06.2019 10:30

English, 22.06.2019 10:30

Mathematics, 22.06.2019 10:30







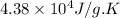
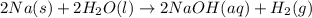
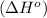 .
.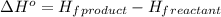
![\Delta H^o=[n_{NaOH}\times \Delta H_f^0_{(NaOH)}+n_{H_2}\times \Delta H_f^0_{(H_2)}]-[n_{Na}\times \Delta H_f^0_{(Na)+n_{H_2O}\times \Delta H_f^0_{(H_2O)}]](/tpl/images/0402/5848/95f1f.png)
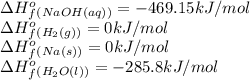
![\Delta H^o_{rxn}=[(2\times -469.15)+(1\times 0)]-[(2\times 0)+(2\times -285.8)]=-652.5kJ](/tpl/images/0402/5848/c5dc6.png)
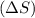 .
.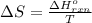
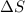 = change in entropy
= change in entropy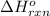 = change in enthalpy = -652.5 kJ
= change in enthalpy = -652.5 kJ