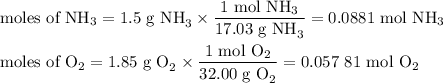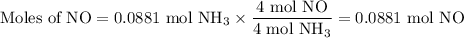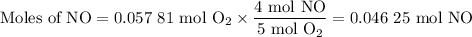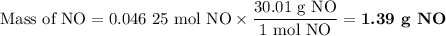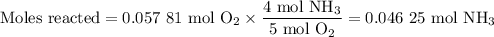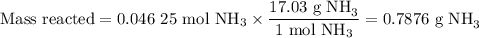no:

Chemistry, 04.12.2019 19:31 ErickP1686
1.) the process for converting ammonia to nitric acid involves the conversion of nh3 to
no:
nh3 + o2 + no + h2o
balanced: 4nh3 +502 uno+ 6h2o
a.) how many grams of no form when 1.5g of nh3 reacts with 1.85g of o2? b.) which
reactant is the limiting reactant and which one is the excess reactant? c.) how much of
the excess reactant remains after the limiting reactant is completely consumed?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
Which sentence best describes the formation of igneous rock? a- lava on the surface dries up and makes arock b_melted rocks cools and forms crystals c_rocks under tremendous heat and pressure d_magma is melted rock underground
Answers: 1

Chemistry, 22.06.2019 02:30
Which compound contains both ionic and covalent bonds? a) hbr b)cbr4 c)nabr d) naoh
Answers: 2

Chemistry, 22.06.2019 05:00
Given sno2 + 2h2 - sn + 2h20 tin oxide reacts with hydrogen to produce tin and water. how many moles of sno2 are needed to produce 500.0 grams of sn?
Answers: 3

Chemistry, 22.06.2019 08:00
An electron moved from shell n = 2 to shell n = 1. what most likely happened during the transition? a fraction of a photon was added. a photon of energy was absorbed. a fraction of a photon was removed. a photon of energy was released.
Answers: 1
You know the right answer?
1.) the process for converting ammonia to nitric acid involves the conversion of nh3 to
no:
no:
Questions




English, 02.01.2021 14:50

Mathematics, 02.01.2021 14:50

Chemistry, 02.01.2021 14:50


Mathematics, 02.01.2021 14:50



English, 02.01.2021 14:50

Computers and Technology, 02.01.2021 15:00

Mathematics, 02.01.2021 15:00

Mathematics, 02.01.2021 15:00

Mathematics, 02.01.2021 15:00


Mathematics, 02.01.2021 15:00

Business, 02.01.2021 15:00

English, 02.01.2021 15:00

English, 02.01.2021 15:00