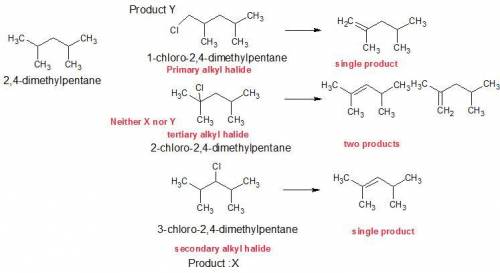
Compounds x and y are both c7h15cl products formed in the radical chlorination of 2,4-dimethylpentane. base-promoted e2 elimination of x and y gives, in each case, a single c7h14 alkene. both x and y undergo an sn2 reaction with sodium iodide in acetone solution to give c7h15i products; in this reaction y reacts faster than x. what is the structure of x?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Which best describes how johannes kepler developed his laws of planetary motion
Answers: 3

Chemistry, 22.06.2019 05:00
Cucl2 + 2nano3 cu(no3)2 + 2nacl what is the percent yield of nacl if 31.0 g of cucl2 reacts with excess nano3 to produce 21.2 g of nacl? 49.7% 58.4% 63.6% 78.7%
Answers: 1

Chemistry, 22.06.2019 06:00
How much would the freezing point of water decrease if 4 mol of sugar were added to 1 kg of water(k=1.86 c/mol/kg for water and i=1 for sugar
Answers: 1

Chemistry, 22.06.2019 13:00
Is 9 correct? and can someone me with 10? it’s due tomorrow, you
Answers: 1
You know the right answer?
Compounds x and y are both c7h15cl products formed in the radical chlorination of 2,4-dimethylpentan...
Questions

Health, 03.12.2021 05:00

Social Studies, 03.12.2021 05:00


Chemistry, 03.12.2021 05:00

Mathematics, 03.12.2021 05:00

Mathematics, 03.12.2021 05:00


Biology, 03.12.2021 05:00


Spanish, 03.12.2021 05:00





Computers and Technology, 03.12.2021 05:00

Mathematics, 03.12.2021 05:00