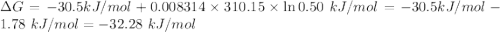Chemistry, 05.12.2019 03:31 chevysilverado3464
For which δg°rxn = –30.5 kj/mol at 37.0 °c and ph 7.0. calculate the value of δgrxn in a biological cell in which [atp] = 5.0 mm, [adp] = 0.50 mm, and [hpo42–] = 5.0 mm.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 19:50
A2.5% (by mass) solution concentration signifies that there is a 2.5 % (by mass) solution concentration signifies that there is blank of solute in every 100 g of solution. of solute in every 100 g of solution
Answers: 3

Chemistry, 23.06.2019 00:30
Balance the following reaction. as2s3 + 9o2 → 2as2o3 + so2
Answers: 2


Chemistry, 23.06.2019 06:50
Organisms are classified as producer or consumers according to the way they ?
Answers: 1
You know the right answer?
For which δg°rxn = –30.5 kj/mol at 37.0 °c and ph 7.0. calculate the value of δgrxn in a biological...
Questions

English, 23.12.2019 04:31


Mathematics, 23.12.2019 04:31




Mathematics, 23.12.2019 04:31



Mathematics, 23.12.2019 04:31


Chemistry, 23.12.2019 04:31

Geography, 23.12.2019 04:31


History, 23.12.2019 04:31


Mathematics, 23.12.2019 04:31

Chemistry, 23.12.2019 04:31



 standard Gibbs energy
standard Gibbs energy
![Q=\frac{[ADP][HPO_4^{2-}]}{[ATP]}](/tpl/images/0403/8875/ccdf0.png)
![[ATP]=5.0 mM](/tpl/images/0403/8875/1ddd2.png)
![[ADP]=0.50 mM](/tpl/images/0403/8875/91d08.png)
![[HPO_4^{2-}]=5.0 mM](/tpl/images/0403/8875/ff97d.png)