
Chemistry, 05.12.2019 05:31 sumayyahjj
How much heat (in kj) is required to warm 13.0 g of ice, initially at -10.0 ∘c, to steam at 111.0 ∘c? the heat capacity of ice is 2.09 j/g⋅∘c and that of steam is 2.01 j/g⋅∘c, the heat of fusion for water is 6.02 kj/mol, and the heat of vaporization for water is 40.7 kj/mol.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:30
Calculate the h3o+ concentration in a solution of acetic acid if the concentration of molecular acetic acid present at equilibrium is 9.97x10^-3 m and k for the dissociation is 1.86x10^-5. ch3cooh(aq)+h2o(> h3o^+(aq)+ch3coo^-(aq)
Answers: 2

Chemistry, 22.06.2019 15:30
What best discribes the relationship between wavelength and frequency in a electromagnetic wave
Answers: 1

Chemistry, 22.06.2019 20:30
Which of the following is not true about the atomic model of substances?
Answers: 1

You know the right answer?
How much heat (in kj) is required to warm 13.0 g of ice, initially at -10.0 ∘c, to steam at 111.0 ∘c...
Questions


Mathematics, 21.09.2019 01:10

Mathematics, 21.09.2019 01:10

History, 21.09.2019 01:10

History, 21.09.2019 01:10



Mathematics, 21.09.2019 01:10


French, 21.09.2019 01:10


Mathematics, 21.09.2019 01:10







Mathematics, 21.09.2019 01:10

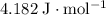 , the melting point of water is
, the melting point of water is 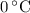 , and that the boiling point of water is
, and that the boiling point of water is 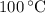 .
.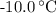 ice to steam at
ice to steam at 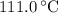 .
.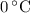 . The change in temperature would be
. The change in temperature would be 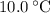 .Step two: supply the heat of fusion to convert that 13.0 gram of ice to water.Step three: heat the 13.0 gram of water from
.Step two: supply the heat of fusion to convert that 13.0 gram of ice to water.Step three: heat the 13.0 gram of water from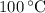 . The change in temperature would be
. The change in temperature would be 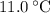 .Energy required for step one, three, and five
.Energy required for step one, three, and five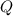 required to raise the temperature of an object by a
required to raise the temperature of an object by a  :
: 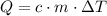 .
.  is the specific heat of this substance,
is the specific heat of this substance, 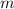 is the mass of the substance, and
is the mass of the substance, and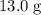 .
.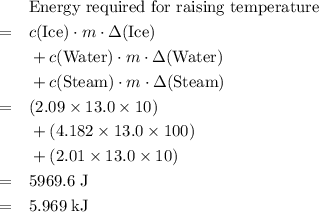 .
.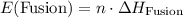 .
.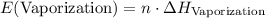 .
.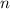 is the number of moles of the substance.
is the number of moles of the substance.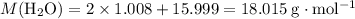 .
.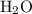 molecules in
molecules in 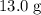 :
: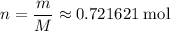 .
. 
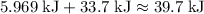 .
.


