
Chemistry, 05.12.2019 05:31 Jcausey4477
A) how many moles of co2 and h2o are formed from 3.85 mole of propane c3h8 (this calculation needs to be done twice-once fro co2 and once for h20.
b) if 0.647 mole of oxygen in used in the burning of propane, how many moles of each of h2o are produced? how many moles of c3h8 are consumed?
the balanced chemical reaction:
1 c3 + 5 o2 = 3co2 + 4 h2o

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Several kinds of bears are found on earth. most bears are brown or black, but one type of bear, the polar bear, is white. what process led to this difference in fur color? explain your answer.
Answers: 1

Chemistry, 22.06.2019 09:00
What is the percentage composition of carbon in the compound ch4
Answers: 1

Chemistry, 22.06.2019 11:00
The diagram below shows the different phase transitions that occur in matter. which arrow represents the transition in which dew is formed?
Answers: 1

Chemistry, 22.06.2019 14:20
You have a liquid that exhibits diltancy. you want to pour it from a bottle. what should you do to the bottle before pouring
Answers: 1
You know the right answer?
A) how many moles of co2 and h2o are formed from 3.85 mole of propane c3h8 (this calculation needs t...
Questions


Health, 03.02.2020 08:56

Mathematics, 03.02.2020 08:56

Mathematics, 03.02.2020 08:56





History, 03.02.2020 08:57


History, 03.02.2020 08:57

English, 03.02.2020 08:57



Mathematics, 03.02.2020 08:57

History, 03.02.2020 08:57

Chemistry, 03.02.2020 08:57


Mathematics, 03.02.2020 08:57

Biology, 03.02.2020 08:57

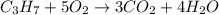
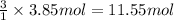 of carbon dioxide gas.
of carbon dioxide gas.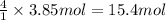 of water .
of water .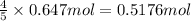 of water.
of water.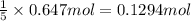 of propane.
of propane.

