when ethane c2h6) burns, it produces carbon dioxide and water:
2c3h5(0) +702(g) → 4co2(g) + 6...

Chemistry, 05.12.2019 09:31 wyattdawes45
when ethane c2h6) burns, it produces carbon dioxide and water:
2c3h5(0) +702(g) → 4co2(g) + 6h2011)
how many liters of carbon dioxide will be produced when 269 l of ethane are
burned? (one mole of any gas occupies 22.4 l under certain conditions of
temperature and pressure. assume those conditions for this question.)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Drag each label to the correct location on the chart. classify each reaction as endothermic or exothermic.
Answers: 1

Chemistry, 22.06.2019 15:00
Areaction is first order with respect to reactant x and second order with respect to reactant y. which statement describes the rate law for this reaction?
Answers: 1

Chemistry, 22.06.2019 17:30
Observation and experimentation have led many scientists to accept a theory about the origin of the universe. this theory is called the big bang theory. scientific evidence collected and observed by scientists around the world suggests that the universe is ever expanding from a hot and dense initial state. what makes this a scientific theory? (2 points)
Answers: 2

Chemistry, 23.06.2019 00:30
What is the percent by mass of magnesium sulfate in mgso4.7h2o
Answers: 3
You know the right answer?
Questions







English, 25.10.2020 07:30

Mathematics, 25.10.2020 07:30

Mathematics, 25.10.2020 07:30

Biology, 25.10.2020 07:30

SAT, 25.10.2020 07:30

World Languages, 25.10.2020 07:30

Biology, 25.10.2020 07:30

Spanish, 25.10.2020 07:30


Mathematics, 25.10.2020 07:30


SAT, 25.10.2020 07:30

Mathematics, 25.10.2020 07:30

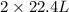 of ethane gives
of ethane gives 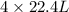 of CO₂.
of CO₂.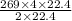
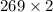 L
L

