
The activation energy for a reaction is changed from 184 kj/mol to 60.5 kj/mol at 600. k by the introduction of a catalyst. if the uncatalyzed reaction takes about 2537 years to occur, about how long will the catalyzed reaction take? assume the frequency factor a is constant and assume the initial concentrations are the same.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:50
Compare the equilibrium constants for the systems shown in the table. which favors products the most? which favors products the least? rank these systems in order from most to least in terms of favoring products rather than reactants. d > b > a > c c > a > b > d b > c > d > a a > d > c > b
Answers: 1

Chemistry, 22.06.2019 05:50
What are transitions between a liquid and gas called? identify which way they are transitioning
Answers: 2

Chemistry, 22.06.2019 17:00
Complete each row of the table below by filling in the missing prefix or missing exponent.
Answers: 1

Chemistry, 22.06.2019 18:20
Categorize them by metal, nonmetal, in periodic tableductilenon-ductilemalleableoften gain electrons easilygood conductorpoor conductorcan be liquidselements
Answers: 2
You know the right answer?
The activation energy for a reaction is changed from 184 kj/mol to 60.5 kj/mol at 600. k by the intr...
Questions

English, 28.04.2021 21:50

Mathematics, 28.04.2021 21:50



Mathematics, 28.04.2021 21:50


Mathematics, 28.04.2021 21:50

Mathematics, 28.04.2021 21:50



Mathematics, 28.04.2021 21:50


English, 28.04.2021 21:50



English, 28.04.2021 21:50


Mathematics, 28.04.2021 21:50


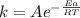
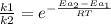
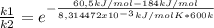
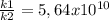
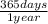 ×
×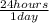 ×
×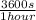 = 1,41 s
= 1,41 s

