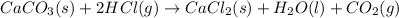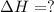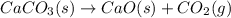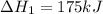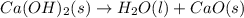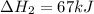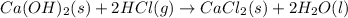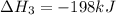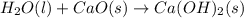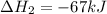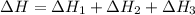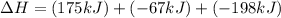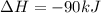Consider the following chemical equations to answer the question that follows. caco3(s)ca(oh)2(s)ca(oh)2(s)+2hcl(g )→cao(s)+co2(g)→h2o(l)+cao(s)→cacl2 (s)+2h2o(l)δhδhδh=175kj=67kj=−198kj using the information above, determine the change in enthalpy for the following chemical reaction. caco3(s)+2hcl(g)⟶cacl2(s)+h2o(l)+co 2(g)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 11:00
3) in peaches, [oh]=3.16x10-11 m a) find [h+ ] b) what is the ph? c) is the solution acidic, basic, or neutral?
Answers: 1

Chemistry, 22.06.2019 12:00
Under normal conditions, describe how increasing the temperatures effects the solubility of a typical salt
Answers: 1

Chemistry, 22.06.2019 14:30
For the reaction shown, find the limiting reactant for each of the following initial amounts of reactants. 4al(s)+3o2(g)→2al2o3(s) a) 1 molal, 1 mol o2 b) 4 molal, 2.6 mol o2 c) 16 molal, 13 mol o2 d) 7.4 molal, 6.5 mol o2
Answers: 3

Chemistry, 22.06.2019 22:30
Which is a characteristic of the electron sea model for metallic bonding? molecular orbitals overlap to produce bands. electrons flow easily between metal nuclei. electrons are in fixed positions in the orbitals. atomic nuclei are arranged in an irregular pattern.
Answers: 3
You know the right answer?
Consider the following chemical equations to answer the question that follows. caco3(s)ca(oh)2(s)ca(...
Questions


Biology, 28.07.2019 14:30



History, 28.07.2019 14:30

History, 28.07.2019 14:30


History, 28.07.2019 14:30


Biology, 28.07.2019 14:30

Social Studies, 28.07.2019 14:30







Social Studies, 28.07.2019 14:30