
Chemistry, 05.12.2019 18:31 madisonruh
Given the reactions, x ( s ) + 1 2 o 2 ( g ) ⟶ xo ( s ) δ h = − 505.9 kj xco 3 ( s ) ⟶ xo ( s ) + co 2 ( g ) δ h = + 199.3 kj what is δ h for this reaction? x ( s ) + 1 2 o 2 ( g ) + co 2 ( g ) ⟶ xco 3 ( s )

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:00
Will give ! what are the advantages and disadvantages of nuclear power? check all that apply. one advantage of nuclear energy is that it does not produce carbon dioxide emissions. storage of nuclear waste is a short-term problem associated with nuclear energy. the problem with uranium mining is that a large quantity of uranium must be extracted to meet energy needs because the energy release from uranium fission is so low. safe operation of a nuclear power plant can be jeopardized by a human mistake.
Answers: 1

Chemistry, 22.06.2019 09:30
In 2002, the rare earth elements mine in mountain pass, california was closed because
Answers: 1

Chemistry, 22.06.2019 19:00
How does a catalyst increase the speed of a reaction? a. the catalyst eliminates the activated complex stage, allowing products to form immediately. b. the catalyst lowers the energy level of the reactants, making it easier for them to react. c. the catalyst makes it easier for the activated complex to form, lowering the activation energy. d. the catalyst raises the energy level of the products, making the reaction finish sooner. reset next
Answers: 1

Chemistry, 22.06.2019 20:30
Citric acid has a ph between 1 and 3. it is considered to be aa. weak acidb. weak basec. strong based. strong acid
Answers: 2
You know the right answer?
Given the reactions, x ( s ) + 1 2 o 2 ( g ) ⟶ xo ( s ) δ h = − 505.9 kj xco 3 ( s ) ⟶ xo ( s ) + co...
Questions





English, 17.08.2021 19:50

Mathematics, 17.08.2021 19:50



Mathematics, 17.08.2021 19:50

History, 17.08.2021 19:50


Mathematics, 17.08.2021 19:50



Computers and Technology, 17.08.2021 19:50


History, 17.08.2021 19:50

Mathematics, 17.08.2021 19:50


History, 17.08.2021 19:50

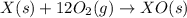
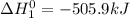 (1)
(1)
 (2)
(2)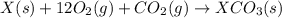
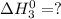 (3)
(3)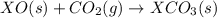
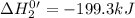 (2')
(2')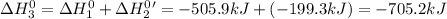
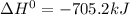 .
.


