
Chemistry, 18.12.2019 03:31 dalechloe5596
Which of the following processes would you predict to have an increase in entropy?
condensation of water
freezing water
melting ice
deposition of co2 (changing from a gas to solid)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:30
You have 125g of a certain seasoning and are told that it contains 76.0 g of salt what is the percentage of salt by mass in this seasoning
Answers: 1

Chemistry, 22.06.2019 04:30
Acamcorder has a power rating of 17 watts. if the output voltage from its battery is 7 volts, what current does it use?units:
Answers: 1


Chemistry, 22.06.2019 12:10
Consider the reaction: n2(g) + o2(g) ⇄ 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3
You know the right answer?
Which of the following processes would you predict to have an increase in entropy?
cond...
cond...
Questions


Mathematics, 25.07.2019 15:30


Physics, 25.07.2019 15:30

Arts, 25.07.2019 15:30


Computers and Technology, 25.07.2019 15:30


History, 25.07.2019 15:30

History, 25.07.2019 15:30





Computers and Technology, 25.07.2019 15:30



Mathematics, 25.07.2019 15:30


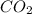 : deposition process where gaseous state changes to solid state by escaping liquid state, thus decreasing randomness and decreasing entropy.
: deposition process where gaseous state changes to solid state by escaping liquid state, thus decreasing randomness and decreasing entropy.

