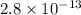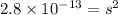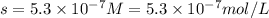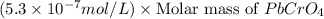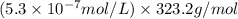Chemistry, 05.12.2019 18:31 BrainlyAvenger
G[mcquarrie 22-7] the value of ksp for pbcro4(s) in equilibrium with water at 25◦c is 2.8 · 10−13m2 . write the chemical equation that represents the solubility equilibrium for pbcro4(s) and calculate its solubility in grams per liter in water at 25◦c.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
If 3.00 g of titanium metal is reacted with 6.00 g of chlorine gas, cl2, to form 7.7 g of titanium (iv) chloride in a combination reaction, what is the percent yield of the product?
Answers: 1

Chemistry, 22.06.2019 02:50
What is the overall order of reaction for rate = k[no2]2 ? second order 3/2 order third order zero order none of the listed answers are correct
Answers: 3

Chemistry, 22.06.2019 05:00
Frictional forces acting on an object are often converted into energy, which causes the temperature of the object to rise slightly.
Answers: 2

You know the right answer?
G[mcquarrie 22-7] the value of ksp for pbcro4(s) in equilibrium with water at 25◦c is 2.8 · 10−13m2...
Questions

Geography, 11.10.2019 00:10


History, 11.10.2019 00:10

Geography, 11.10.2019 00:10



Geography, 11.10.2019 00:10









Mathematics, 11.10.2019 00:10




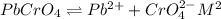
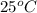 is,
is, 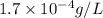
![K_{sp}=[Pb^{2+}][CrO_4^{2-}]](/tpl/images/0404/8064/04f28.png)
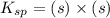
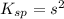
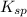 =
=