13. what is the molecular composition of the following compound: iodine heptafluoride. *
1 po...

Chemistry, 05.12.2019 19:31 namirah0303
13. what is the molecular composition of the following compound: iodine heptafluoride. *
1 point
six iodine atoms and two fluorine atoms
seven iodine atoms and one fluorine atom
one iodine atom and seven fluorine atoms
two iodine atoms and six fluorine atoms

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:10
Which class of molecules functions as chemical signals? hormones water carbohydrates proteins
Answers: 1

Chemistry, 22.06.2019 10:50
Someone offer some answers to this, i will give 98 coins and mark as brainliest! i will put the rest of the lab down in the comments,solutions pre-lab questions: in this lab, you will make fruit drinks with powdered drink mix. complete the pre-lab questions to get the values you need for your drink solutions. calculate the molar mass of powered fruit drink mix, made from sucrose (c12h22o11).using stoichiometry, determine the mass of powdered drink mix needed to make a 1.0 m solution of 100 ml. (hint: use molarity = to find the moles of drink mix, then convert moles to grams using a mole conversion.)what mass of powdered drink mix is needed to make a 0.5 m solution of 100 ml?
Answers: 1


Chemistry, 22.06.2019 16:00
Which process transfers heat from inside earth to its surface? convection currents in mantle pulling away of tectonic plates drawing in of tectonic plates convection currents in crust
Answers: 1
You know the right answer?
Questions


Mathematics, 04.03.2021 23:30






History, 04.03.2021 23:30


Mathematics, 04.03.2021 23:30


Mathematics, 04.03.2021 23:30



Mathematics, 04.03.2021 23:30

English, 04.03.2021 23:30

Biology, 04.03.2021 23:30


Mathematics, 04.03.2021 23:30

Chemistry, 04.03.2021 23:30

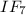 and it is also known as Iodine (VII)fluoride.
and it is also known as Iodine (VII)fluoride.

